Journal of Caring Sciences. 13(3):158-166.
doi: 10.34172/jcs.33228
Original Article
Translation and Validation of the Functional Assessment of Chronic Illness Therapy-Palliative Care (Facit-Pal-14) Scale
Ioanna Tsatsou Data curation, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing, 1 
Efi Parpa Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing, 2
Maria Nikoloudi Resources, Visualization, Writing – original draft, 2 
Maria-Aggeliki Kalogeridi Data curation, Investigation, Resources, Visualization, 3
Euaggelia Keramida Data curation, Investigation, Resources, 4
Antonis Galanos Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, 5 
Kyriaki Mystakidou Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing, 2, * 
Author information:
1One Day Clinic, Hellenic Airforce General Hospital, Athens, Greece
2Pain Relief and Palliative Care Unit, Department of Radiology, Areteion Hospital, School of Medicine, National & Kapodistrian University of Athens, Athens, Greece
3Radiotherapy Department, Alexandra General Hospital, Athens, Greece
4Radiotherapy Department, 401 General Army Hospital, Athens, Greece
5Laboratory for Research of the Musculoskeletal System, National & Kapodistrian University of Athens, Kifissia, Greece
Abstract
Introduction:
In palliative care, assessing outcomes and evaluating quality of life (QoL) are essential to ensure high-quality, evidence-based care. The aim of this study was the Greek validation of the Functional Assessment Chronic Illness Therapy-Palliative Care (FACIT-PAL-14) in patients with cancer.
Methods:
The FACIT-PAL-14 was translated into Greek and administered to 185 patients with cancer treated in two central hospitals of Athens, Greece. Data collection lasted from January to March 2022. FACIT-PAL-14 is a 41 item measurement of QoL that includes the 27 items of the FACIT-General and 14 additional items that form the palliative care scale. The Monroe Dunaway Anderson Symptom Inventory (MDASI), was used to evaluate the criterion validity. Also the following analyses were conducted; confirmatory factor analysis (CFA), exploratory factor analysis (EFA), concurrent validity, internal consistency and instrument stability.
Results:
Participants’ mean (SD) age was 57.37(14.38) and the majority were women (55.1%) and had breast cancer (31.4%). Three factors were exported from the statistical analysis of the palliative care scale that explained the 62.21% of the variance. Τhese factors were psychological wellbeing, physical symptoms and close relationships. FACIT-PAL-14 and its factors had high internal consistency. Cronbach’s alpha coefficient for the total score of the FACIT-PAL-14 questionnaire was 0.781. Intraclass correlation (ICC) between initial assessment and reassessment of the FACIT-PAL-14 factor 1, factor 2, factor 3 and total score were 0.985, 0.972, 0.981 and 0.991 respectively. FACIT-PAL-14 subscales presented moderate correlation with MDASI subscales.
Conclusion:
The Greek version of FACIT-PAL-14 is valid and reliable scale in patients with cancer.
Keywords: Palliative care, Quality of life, Cancer, Patients, Translation, Validation
Copyright and License Information
© 2024 The Author(s).
This work is published by Journal of Caring Sciences as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
Νοne.
Introduction
Τhe World Health Organization defines palliative care as “an approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual”. Palliative care enhances the quality of life of people affected by chronic diseases and has a positive influence at the course of illness.1 In cancer care, it is offered together with other treatments, such as chemotherapy or radiotherapy and includes a better understanding and management of the clinical manifestations of cancer and its treatments that cause distress to patients.2
Quality of life (QoL) is also defined by the World Health Organization as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns”.3 The concept of QoL is broad and complex and it is influenced by many factors. These include the physical and mental state of a person, its social interactions and social environment and its level of independence. Ultimately, QoL is a multidimensional concept that covers a broad range of content, including physical, functional, emotional, and social well-being and a subjective concept that is interpreted and defined by each individual.4
For patients with cancer, QoL is of a great value because the disease itself combined with treatments’ toxicity cause great burden and distress to them.5 Advances in the QoL assessments over the past 30 years have allowed healthcare professionals to address the challenge of effectively measuring QoL in patients with cancer.6
In palliative cancer care, assessing outcomes and evaluating the care are essential to ensure high-quality, evidence-based care. Palliative care assessment by oncology teams includes evaluation of the benefits and risks of treatments, assessment of physical symptoms, psychosocial and spiritual distress, educational and informational needs, personal goals and hopes and cultural aspects affecting care.7
For measuring the QoL of people with chronic illnesses, the FACIT measurement system has a set of multidimensional instruments. These instruments are managed and officially distributed by the FACIT organization.8 The Functional Assessment of Chronic Illness Therapy-General (FACT-G), is a 27-item general measure of health-related QoL in patients with chronic diseases. It includes four scales: physical, social, emotional and functional wellbeing. In addition to the general scale (FACT-G), there are currently over 50 subscales available in English that are disease specific and treatment or symptom specific.9 The FACIT-PAL instrument is comprised of the FACT-G plus the palliative care subscale (19 items), that were reported via interviews with advanced cancer patients.10The FACIT-PAL-14 is the shorten version of the FACIT-PAL, with 14 items in the palliative care subscale and was created after interviews with 60patients and 56 healthcare professionals.11
There is a wide variety of tools for assessing patients’ QoL in patients with cancer.12 It should be noted that there is no “gold standard” tool for the assessment of QoL and this makes its evaluation even more difficult.13 For the assessment of QoL in palliative care patients, the EORTC-QLQ-C15-PAL (European Organization for Research and Treatment of Cancer (EORTC), the Palliative Care Outcome Scale (POS) have been used14; widely by palliative care specialists in Europe.15 Also, other tools such as the EORTC-QLQ-C30, the McGill QOL questionnaire and the EQ-5D have been validated in the palliative care setting.14
FACIT-PAL and its shortened version have undergone little psychometric evaluation to date, despite the fact that it is concise and easy to use, and could be applied in different cultural populations. Until today it has not been translated and validated in the Greek language. Thus, the purpose of the present study was the translation and validation of the FACIT-PAL-14 in Greek language.
Materials and Methods
A prospective, observational, cross-sectional, non-randomized, study was conducted in which measurement scales were used for data collection and multiple methods of data analysis. Data collection was carried out from January to March 2022.
The study was carried out in two central public hospitals in Athens, Greece. Participants were selected from cancer patients attending the oncology outpatient, inpatient and radiotherapy unit. The sample was collected through convenience sampling and consisted of 185 patients with cancer. Patients that were included in the study were adults (age of 18 or older) with any cancer type and any stage and had the ability to provide verbal informed consent and understanding and reading of the Greek language (native language). Patients who did not understand the Greek language sufficiently and those who suffered from a psychiatric disorder were excluded from participating in the study.
Patients completed the Functional Assessment of Chronic Illness Therapy-Palliative Care (FACIT-PAL-14), the Monroe Dunaway Anderson Symptom Inventory (MDASI), along with demographic and clinical data. The construct validity of the FACIT-PAL-14 was tested by performing correlation analysis between the FACIT-PAL-14 and the MDASI subscales. The time for completing the questionnaires was approximately 20 minutes.
The FACIT-PAL-14 is the shortened 14-item questionnaire of the FACIT-PAL that has been generated for the palliative care population. It is a self-report questionnaire for evaluating the QoL in palliative care patients. It consists of 41 items that are divided into five subscales: physical well-being (seven items), social/family well-being (seven items), emotional wellbeing (six items), and functional well-being (seven items) and one that collects other additional concerns (14 items) that form the palliative care subscale (Pal). Per se, the FACIT-PAL contains the 27-items of the FACT-G and adds 14 items. Items are rated using a five-point Likert-type (0-4) scale (0 not at all, 1 a little bit, 2 somewhat, 3 quite a bit, 4 very much). The recall period is the past week. For the FACIT-PAL-14, scores range from 0 to 56 for the palliative care subscale and from 0 to 164 for the whole instrument. Higher scores indicate a better QoL.11
The MDASI is used to assess the presence and severity of cancer-related symptoms experienced by patients with cancer and their impact in daily living in the last 24 hours. The questionnaire consists of two parts. The core MDASI (part I), which consists of 13 symptom items and rated based on their presence and severity. Each symptom is rated on an 11-point numeric scale ranging from 0 (not present) to 10 (as bad as you can imagine). In part II of the questionnaire, patients rate the degree to which symptoms interfere with their daily living (general activity, mood, work, relations with other people, walking and enjoyment of life). These range from 0 (did not interfere) to 10(interfered completely). The Greek version of the MDASI is translated and validated by Mystakidou et al.16
Patients’ performance status was assessed with the Eastern Cooperative Oncology Group (ECOG) scale. The 0 stands for functional status with normal activity without limitation, as before the disease, 1 for limitation of vigorous physical activities, but normal mobility and ability for light work, 2 for ability only for self-care, inability for any work, 3 for ability of only limited self-care and 4 for complete inability of self-care.17
According to instructions given from the FACIT organization, translation and linguistic validation methodology, at first two independent bilingual researchers made two forward translations from English to the Greek language. Afterwards, a union of these two forward translations was provided by a third translator. Finally, a back translation into English was performed by a fourth translator and the final translation was reviewed and finalized by a fifth translator. Then, the final version was tested on 10 patients. They completed the questionnaire and answered questions from the cognitive debriefing script that was prepared from the FACIT organization and translated by the research team. The interviews lasted up to 30 minutes, of which 10 min were required for most of the patients to complete the FACIT-PAL-14. The items of the questionnaire were not found to be irrelevant, upsetting or disturbing for any patient.
Permission for the use of the FACIT-PA-14L was requested through the official website FACIT Measurement System.
The research was also carried out after permission had been obtained from the hospitals’ ethics and university research committee. Patients were informed verbally and written about anonymity, confidentiality, voluntary participation, the possibility to withdraw from the study at any time and signed the consent form. Then, the participants answered, at the same time, the questionnaire in the presence of the researcher, after thorough explanation. In addition, protection of the participants’ personal data was ensured by anonymous completion of questionnaires and code assignment.
The confirmatory factor analysis (CFA) was used to examine the factor structure of the questionnaire as suggested by the creator of the questionnaire. The CFA was carried out using the Analysis of Moment Structure (AMOS) Version 21.0. The sample size required for the CFA based on researcher’s conventions ranging for the participant’s ratio 3:1 to as high as 12:1. The FACIT-PAL 14 consisted of 14 items, thus our sample size of 185 is within the above guidelines.
Rejecting or accepting a model was based on some global fit indices; (1) chi-square-degrees of freedom (d. f.) ratio (2) the root mean square error of approximation (RMSEA); (3) the comparative fit index (CFI); (4) the normed fit index (NFI); (5) the goodness fit index (GFI) and; (6) the adjusted GFI (AGFI) The chi-square-degrees of freedom (df) ratio < 2.0,18RMSEA < 0.08, CFI > 0.90,19GFI > 0.85, AGFI > 0.80,20 NFI > 0.90,21indicate an acceptable fit.
Exploratory factor analysis (EFA) was conducted using maximum likelihood extraction method with Varimax rotation, was conducted for all participants to determine the factor structure of the 14 items of the FACIT-PAL-14 questionnaire. The selection of factors was based on the following criteria (a) eigenvalues ≥ 1; (b) items with factor loadings ≥ 0.4; (c) items that did not load on more than one factor. The numbers factors to retain were also confirmed by using a Monte Carlo PCA.
Construct validity of the translated version of the FACIT-PAL-14 was assessed by establishing its correlation to the MDASI subscales using the Pearson’s correlation coefficient. Moderate or high correlation between FACIT-PAL-14 to a well-established questionnaire would support the validity of the FACIT-PAL-14 questionnaire in measuring QoL in the palliative setting.
Floor or ceiling effects are considered to be present if more than 15% of respondents achieved the lowest or highest possible score, respectively.
The internal consistency reliability of the FACIT-PAL-14 was determined by calculating the Cronbach alpha coefficient. A Cronbach alpha coefficient value of 0.7 indicates sufficient reliability for research purposes and suggests that items are interdependent and homogeneous in terms of the construct they measure. For clinical applications it is desirable to have a Cronbach alpha above 0.8.22
Test-retest reliability indicates the stability of patients’ response in time and it was determined by calculating the intraclass correlation coefficient (ICC) between the initial assessment of the FACIT-PAL-14 and the reassessment after 3 days. In test-retest situations, studies follow the appropriate design for analysis by means of two-way models. A two-way random effects model to measure agreement, which assumes that the different assessments are randomly selected, is appropriate for most test-retest evaluations in QoL studies.23
All tests were two-sided, a P value of < 0.05 was used to denote statistical significance. All analyses were carried out using the statistical package SPSS version 21.00 (IBM Corporation, Somers, NY, USA).
Results
Descriptive Characteristics
The mean (SD) age of the 185 patients with cancer of the sample was 57.37 (14.38) (range 18-87 years). The majority of the participants were women (55.1%), married (64.3%), university graduates (45.9%) and employed (43.2%). Their functional status was ECOG 0 at 52.4 % and 31.4% had breast cancer. Most of the cancers were at stage 2 (30.3%) with no metastasis (69.7%). Finally, regarding the treatments that patients received, 78.4% had received chemotherapy, 82.2% radiotherapy and 61.6% surgery treatment (Table 1).
Table 1.
Descriptive statistics of participants
|
Patients
|
Νo. (%)
|
| Gender |
Women |
102 (55.1( |
| Men |
83 )44.9( |
| Ethnicity |
Greek |
175 (94.6) |
| Other |
10 (5.4) |
| ECOG |
0 |
97 (52.4) |
| 1 |
66 (35.7) |
| 2 |
18 (9.7) |
| 3 |
4 (2.2) |
| Marital Status |
Unmarried |
27 (14.6) |
| Married |
119 (64.3) |
| Divorced |
18 (9.7) |
| Widowed |
21 (11.4) |
| Educational level |
Elementary school |
27 (14.6) |
| High school |
73 (39.5) |
| University |
85 (45.9) |
| Employment |
Employed |
80 (43.2) |
| Unemployed |
21 (11.4) |
| Retired |
66 (35.7) |
| Cancer diagnosis |
Lung |
35 (18.9) |
| Breast |
58 (31.4) |
| Gynecological |
16(8.6) |
| Urinary |
26 (14.1) |
| Gastrointestinal |
16 (8.6) |
| Other |
34 (18.4) |
| Disease stage |
I |
43 (23.2) |
| II |
56 (30.3) |
| III |
51 (27.6) |
| IV |
35 (18.9) |
| Metastasis |
Yes |
56 (30.3) |
| No |
129 (69.7) |
| Comorbidities |
Yes |
89 (48.1) |
| No |
96 (51.9) |
| Chemotherapy |
Yes |
145 (78.4) |
| Radiotherapy |
152 (82.2) |
| Surgery |
|
114 (61.6) |
| Age |
Μean )SD( |
57.37 (14.38) (range 18-87) |
The Validation of the FACIT-PAL-14 Questionnaire
The Greek version of FACIT-PAL-14 was assessed in terms of its validity and reliability.
Confirmatory Factor Analysis (CFA) Original
A one-factor model of FACIT proposed by the creator, was examined by CFA giving unacceptable global fit indices. The resulting global fit indices chi-square-degrees of freedom (d.f.) ratio = 6.98, RMSEA = 0.180, CFI = 0.520, NFI = 0.486, GFI = 0.548, AGFI = 0.439 showed that the one factor solution should be rejected.
Exploratory Factor Analysis (EFA)
The Kaiser-Meyer-Olkin Measure of Sampling Adequacy was equal to 0.870 showing suitable data for factor analysis. The hypothesis of no intercorrelation of items was rejected by Bartlett’s test of sphericity (χ2 = 1996.3 df = 171, P < 0.001.
The 14 items were analyzed via maximum likelihood extraction method using a Varimax rotation. Three factors, with eigenvalue of over 1 and loadings ≥ 0.40 were identified. The eigenvalue for the first factor was 6.62, explaining 34.9% of the variance. Factor loadings, ranged from 0.587 to 0.850 for the Factor 1. The eigenvalue for the second factor was 3.62, explaining 19.1% of the variance. Factor loadings, ranged from 0.497 to 0.793 for the Factor 2. The eigenvalue for the third factor was 1.58, explaining 8.3% of the variance. Factor loadings, ranged from 0.649 to 0.832 for the Factor 3 (Tables 2 and 3).
Table 2.
Eigenvalues and explained variance of FACIT-PAL-14 questionnaire
|
Factors
|
Eigen values
|
% of Variance
|
Cumulative %
|
| 1 |
6.623 |
34.86 |
34.86 |
| 2 |
3.618 |
19.04 |
53.90 |
| 3 |
1.579 |
8.31 |
62.20 |
| 4 |
0.917 |
4.83 |
67.03 |
| 5 |
0.741 |
3.90 |
70.94 |
| 6 |
0.696 |
3.66 |
74.60 |
| 7 |
0.673 |
3.54 |
78.14 |
| 8 |
0.605 |
3.19 |
81.33 |
| 9 |
0.544 |
2.87 |
84.19 |
| 10 |
0.512 |
2.69 |
86.88 |
| 11 |
0.428 |
2.25 |
89.14 |
| 12 |
0.368 |
1.94 |
91.08 |
| 13 |
0.343 |
1.81 |
92.88 |
| 14 |
0.322 |
1.70 |
94.58 |
| 15 |
0.240 |
1.26 |
95.84 |
| 16 |
0.228 |
1.20 |
97.04 |
| 17 |
0.207 |
1.09 |
98.13 |
| 18 |
0.197 |
1.04 |
99.17 |
| 19 |
0.158 |
0.83 |
100.00 |
Table 3.
Factor structure and loadings of FACIT-PAL-14
|
Items
|
Factors
|
|
1
|
2
|
3
|
| Pal1 |
|
|
0.649 |
| Pal2 |
|
|
0.816 |
| Pal3 |
|
|
0.832 |
| Pal4 |
|
0.618 |
|
| B1 |
|
0.497 |
|
| Pal5 |
|
0.591 |
|
| C2 |
|
0.771 |
|
| O2 |
|
0.793 |
|
| Pal6 |
|
0.739 |
|
| Pal7 |
|
0.706 |
|
| Br7 |
0.691 |
|
|
| Pal8 |
0.700 |
|
|
| Pal9 |
0.850 |
|
|
| Pal10 |
0.650 |
|
|
| Sp21 |
0.766 |
|
|
| Pal12 |
0.811 |
|
|
| L1 |
0.763 |
|
|
| Pal13 |
0.607 |
|
|
| Pal14 |
0.587 |
|
|
Note: Extraction method: maximum likelihood.
Rotation method: Varimax.
Only loadings > 0.4 were presented.
The scree test and Monte Carlo PCA for parallel analysis (the criterion value of forth eigenvalue was 1.32, higher than eigenvalue of the fourth factor of our data which was 0.92) indicated a three factor structure (Figures 1 and 2).
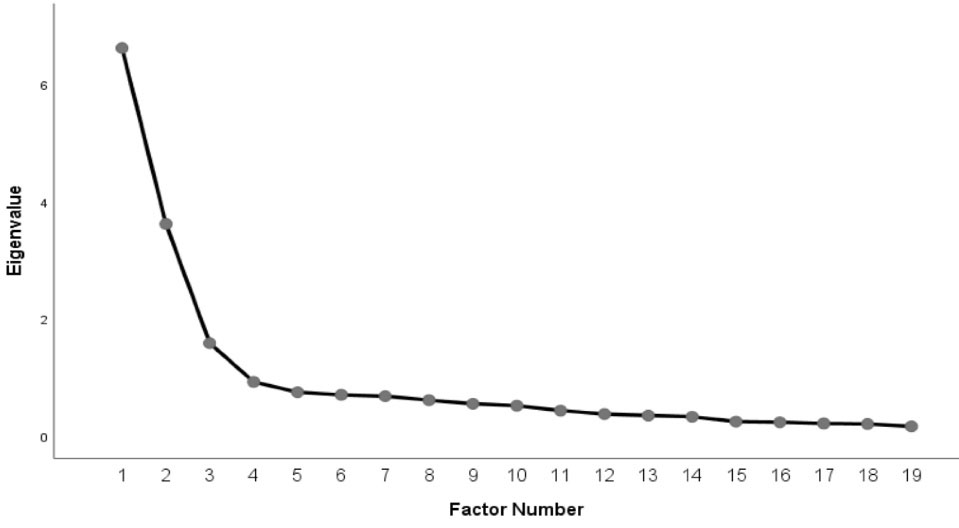
Figure 1.
Scree plot of FACIT-PAL-14 questionnaire; three factors, with eigenvalue of over 1 and loadings ≥ 0.40 were identified. The scree test and Monte Carlo PCA for parallel analysis (the criterion value of forth eigenvalue was 1.32, higher than eigenvalue of the fourth factor of our data which was 0.92) indicated a three factor structure
.
Scree plot of FACIT-PAL-14 questionnaire; three factors, with eigenvalue of over 1 and loadings ≥ 0.40 were identified. The scree test and Monte Carlo PCA for parallel analysis (the criterion value of forth eigenvalue was 1.32, higher than eigenvalue of the fourth factor of our data which was 0.92) indicated a three factor structure
Figure 2.
Confirmatory factor analysis of FACIT-PAL-14 questionnaire; the scree test and Monte Carlo PCA for parallel analysis (the criterion value of forth eigenvalue was 1.32, higher than eigenvalue of the fourth factor of our data which was 0.92) indicated a three factor structure
.
Confirmatory factor analysis of FACIT-PAL-14 questionnaire; the scree test and Monte Carlo PCA for parallel analysis (the criterion value of forth eigenvalue was 1.32, higher than eigenvalue of the fourth factor of our data which was 0.92) indicated a three factor structure
Criterion Validity
Table 4, presents the correlations between the three factors of FACIT-PAL-14 and the MDASI subscales. All Pearson’s correlation coefficients were statistically significant (P < 0.001). Factor 1 had a high negative correlation with the MDASI subscales and there was a moderate positive correlation between factor 2 and both the MDASI subscales. Also, factor 3 had a low negative correlation with the MDASI subscales.
Table 4.
Convergent validity of FACIT-PAL-14 questionnaire
|
|
MDASI-I
|
MDASI-II
|
| Factor 1 |
-0.632 |
-0.657 |
| Factor 2 |
0.400 |
0.351 |
| Factor 3 |
-0.185 |
-0.258 |
| Total Score |
-0.280 |
-0.354 |
Note: All correlations were statistically significant (P < 0.01).
Overall, the total score of FACIT-PAL-14 was moderately and negatively correlated with the MDASI subscales. So, since there is a moderate correlation between the FACIT-PAL-14 and MDASI, the validity of it that FACIT-PAL-14 is supported and it measures QoL in palliative care patients.
Reliability
The reliability of the FACIT-PAL-14 questionnaire was tested for the characteristics of stability and internal consistency.
Internal Consistency
For testing the internal consistency of the FACIT-PAL-14 Cronbach’s alpha coefficient was used. Cronbach’s alpha coefficient for the total score of the FACIT-PAL-14 questionnaire was 0.781 which showed that the scale has very good internal consistency. Furthermore, results produced the following coefficients Cronbach’s a, 0.914 for Factor 1, 0.861 for Factor 2 and 0.847 for Factor 3, indicating that there was high internal consistency.
Test-Retest Method
From the total of 185 patients, 30 of them completed the questionnaire for a second time (retest) after a three-day period. ICC between initial assessment and reassessment of the FACIT-PAL-14 factor 1, factor 2, factor 3 and total score were 0.985, 0.972, 0.981 and 0.991 (P < 0.001) respectively. The above results of stability indicated that MBI factor 1, factor 2, factor 3 and total score were remarkably consistent between the two occasions.
Floor/Ceiling Effect Analysis
Floor and ceiling effect of the 3 factors and total score were presented in Table 5. The critical value of 15% was surpassed only for Factor 3 presenting 26.5% floor effect.
Table 5.
Floor and ceiling effect of FACIT-PAL-14 questionnaire
|
|
Floor effect
|
Ceiling effect
|
| Factor 1 |
0.5% |
12.4% |
| Factor 2 |
7.6% |
1.1% |
| Factor 3 |
4.3% |
26.5% |
| Total score |
0.5% |
0.5% |
Discussion
The aim of our study was to assess the validity and reliability of the Greek version of the FACIT-PAL-14 questionnaire. Overall, the analyses conducted provide evidence to support the internal consistency reliability and validity of the palliative care subscale for use in this population.
Up to date, the FACIT-PAL is translated in four languages, Turkish,24Spanish,25African,26 and German27; but validated only for the first three languages. There is also a study of transcultural adaptation in Colombian advanced cancer patients.28
As indicated from the analysis, the alpha coefficient for the Greek version of the palliative care subscale (α = 0.781) is similar to the alphas reported at the original version (α = 0.82),29 and other FACIT-PAL validation studies, per se, the Turkish (α = 0.860),24African (α = 0.81),26 and the Spanish (α = 0.751) version.25
In addition, no studies were found to evaluate the criterion validity of this scale similar to our study. Other FACIT-PAL validity studies used the Edmonton Symptom Assessment Scale (ESAS),24,29 the Center for Epidemiological Studies-Depression (CES-D),29 and the EORTC-QLQ-C15-PAL.25We used another widely used symptom assessment scale, the MDASI scale to evaluate the criterion validity.
The factor analysis of the 14 items of the palliative care subscale in Greek, revealed three factors, psychological wellbeing, physical symptoms and close relationships. In the original study by Lyons et al29 the scree plot suggested five factors. The four factors were the factors from FACT-G (Physical Well-Being, Functional Well-Being, Emotional Well-Being, Social Well-Being) and two palliative care subscale items (“I am able to make decisions” and “My thinking is clear”) formed a fifth factor, “Clarity of Thought and Decisions”. The Spanish25 and the Turkish24 analysis revealed that the 46 item FACIT-PAL had a structure of five factors (Physical Well-Being, Functional Well-Being, Emotional Well-Being, Social Well-Being, Additional concerns). Finally, at the study of Siegert et al26 in three African countries (Uganda, Kenya, Republic of South Africa), the 19-item FACIT-PAL scale showed three factors, factor 1, a sense of purpose and meaning in life, factor 2, physical symptoms and factor 3, social integration.
As it is noted, our results are somewhat different from the other studies, except the African one. This could be attributed to the cultural differences or to the fact that we chose to factor analyze only the 14 items of the palliative care scale of the FACIT-PAL-14 and not the whole scale as most of the researchers. It is proposed by FACIT that when using the FACIT-PAL, the FACT-G should be summed into the original subscales and the palliative care scale items should be reported separately. Both analytic strategies are valid, with the former focusing on what the measures have in common and our approach focusing on their unique aspects.26
Αt this point the 3 factors of the Greek version of the FACIT-PAL-14 should be given importance. Regarding the physical symptoms, it is well acknowledged that patients with cancer endure a variety of physical symptoms caused by cancer itself and the different treatment approaches.30Palliative cancer patients more often experience pain, breathlessness, fatigue, insomnia, nausea and vomiting, constipation and loss of appetite. When they are not managed appropriately, these symptoms can have a negative impact on patients’ functionality and QoL.31 As indicated from the analysis the seven items that form the factor of physical symptoms include the symptoms of weakness, breathlessness, constipation, weight loss vomiting, swelling, xerostomia. All of these symptoms should be assessed by using patient-reported outcome measures, such as the FACIT-PAL-14. This is an effective approach to improve symptom control.32,33
Afterwards, the other factor was the psychological well-being. Palliative cancer patients have great distress, anxiety, depression and adjustment issues.34,35 Depression, anxiety and distress severely impact patients’ with cancer QoL.36Nevertheless, it is difficult for palliative care doctors to identify, assess and treat the psychological and psychiatric morbidity of patients37; and a minority of patients with psychological distress are treated with psychological interventions.38 The use of validated tools for QoL that assess patients’ patients with cancer psychological wellbeing is encouraged.39 Also there is evidence that the implementation of psychological interventions in palliative care is effective in reducing psychological morbidity. Such interventions include cognitive behavioral-based, mindfulness-based, meaning-based and dignity-based interventions. The research in the field of psychological interventions in palliative care settings is growing and showing promising results.35
The factor of close relationships, included 3 items; “I keep in touch with my friends”, “I have family members who will take over responsibilities for me”, “I feel that my family values me”. The social network of a patient with cancer (family and friends) is a major component of palliative care. Social difficulties that may occur during the palliative care trajectory include relational, family, domestic, communication, financial and legal problems.35 Therefore, palliative cancer patients should be offered sufficient social support and psychosocial care.40 Social support is the perceived availability, or actual provision of information or assistance, that enables a person to manage their daily life effectively. Social support comprises of structural and functional measures. Structural measures include patient’s social relationships and are correlated with their QoL. The functional measures are what is widely considered as social support and include the resources and services provided by people who are involved the patient’s social network and also emotional and informational support.41
The present study is unique in that it assesses the reliability and validity of a new scale not previously thoroughly examined. Also, the FACIT-PAL-14 includes items on physical, emotional, social/family, and functional well-being and symptoms stated to be important for cancer patients and highly related to their QoL. It is short and easy to administer and has been used in different cultures and settings. This Greek validation applies to cancer patients that speak the Greek Language. Νevertheless, this shortened version of the FACIT-PAL needs further psychometric validation.
Conclusion
In summary, the Greek version of the FACIT-PAL-14 is a reliable and valid measure to use in palliative care cancer patients. Future evaluation of the psychometric properties of the Greek version in FACIT-PAL-14 should include diverse samples of patients with other chronic diseases. It is of great importance for healthcare professionals to constantly and effectively assess patients’ QoL, in order to tailor interventions and achieve quality results, throughout the cancer trajectory.
Acknowledgments
We extend our appreciation to the patients who willingly participated in the research, providing valuable insights and data.
Competing Interests
The authors have no conflicts of the interest to declare.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.
Ethical Approval
The research was carried out after permission had been obtained from the hospitals’ ethics and university research committee. Patients were informed verbally and written about anonymity, confidentiality, voluntary participation, the possibility to withdraw from the study at any time and signed the consent form. Protection of the participants’ personal data was ensured by anonymous completion of questionnaires and code assignment.
Research Highlights
What is the current knowledge?
What is new here?
References
- World Health Organization (WHO). National Cancer Control Programmes [Internet]. Geneva: WHO; 2002. Available from: https://www.who.int/publications/i/item/national-cancer-control-programms. Accessed June 20, 2024.
- Sepúlveda C, Marlin A, Yoshida T, Ullrich A. Palliative care: the World Health Organization’s global perspective. J Pain Symptom Manage 2002; 24(2):91-6. doi: 10.1016/s0885-3924(02)00440-2 [Crossref] [ Google Scholar]
- The WHOQOL Group . . Psychol Med 1998; 28(3):551-8. doi: 10.1017/s0033291798006667 [Crossref]
- Cella DF. Quality of life: concepts and definition. J Pain Symptom Manage 1994; 9(3):186-92. doi: 10.1016/0885-3924(94)90129-5 [Crossref] [ Google Scholar]
- Stickel A, Goerling U. Quality of life in oncology. Recent Results Cancer Res 2018; 210:163-80. doi: 10.1007/978-3-319-64310-6_10 [Crossref] [ Google Scholar]
- Cella D, Stone AA. Health-related quality of life measurement in oncology: advances and opportunities. Am Psychol 2015; 70(2):175-85. doi: 10.1037/a0037821 [Crossref] [ Google Scholar]
- Levy MH, Adolph MD, Back A, Block S, Codada SN, Dalal S. Palliative care. J Natl Compr Canc Netw 2012; 10(10):1284-309. doi: 10.6004/jnccn.2012.0132 [Crossref] [ Google Scholar]
- FACIT Group. FACIT Measures & Searchable Library. Available from: https://www.facit.org/facit-measures-searchable-library. Accessed June 20, 2024.
- Cella D, Nowinski CJ. Measuring quality of life in chronic illness: the functional assessment of chronic illness therapy measurement system. Arch Phys Med Rehabil 2002; 83(12 Suppl 2):S10-7. doi: 10.1053/apmr.2002.36959 [Crossref] [ Google Scholar]
- Greisinger AJ, Lorimor RJ, Aday LA, Winn RJ, Baile WF. Greisinger AJ, Lorimor RJ, Aday LA, Winn RJ, Baile WFTerminally ill cancer patientsTheir most important concerns. Cancer Pract 1997; 5(3):147-54. [ Google Scholar]
- Zeng L, Bedard G, Cella D, Thavarajah N, Chen E, Zhang L. Preliminary results of the generation of a shortened quality-of-life assessment for patients with advanced cancer: the FACIT-Pal-14. J Palliat Med 2013; 16(5):509-15. doi: 10.1089/jpm.2012.0595 [Crossref] [ Google Scholar]
- Adamakidou T, Kalokerinou A. Quality of life and cancer patient (Part II): instruments for its assessments. Balkan Military Medical Review 2012; 15(1):47-56. [ Google Scholar]
- Aaronson NK, Meyerowitz BE, Bard M, Bloom JR, Fawzy FI, Feldstein M. Aaronson NK, Meyerowitz BE, Bard M, Bloom JR, Fawzy FI, Feldstein M, et alQuality of life research in oncologyPast achievements and future priorities. Cancer 1991; 67(3 Suppl):839-43. [ Google Scholar]
- Davis MP, Hui D. Quality of life in palliative care. Expert Rev Qual Life Cancer Care 2017; 2(6):293-302. doi: 10.1080/23809000.2017.1400911 [Crossref] [ Google Scholar]
- Harding R, Simon ST, Benalia H, Downing J, Daveson BA, Higginson IJ. Harding R, Simon ST, Benalia H, Downing J, Daveson BA, Higginson IJ, et alThe PRISMA Symposium 1: outcome tool useDisharmony in European outcomes research for palliative and advanced disease care: too many tools in practice. J Pain Symptom Manage 2011; 42(4):493-500. doi: 10.1016/j.jpainsymman.2011.06.008 [Crossref] [ Google Scholar]
- Mystakidou K, Cleeland C, Tsilika E, Katsouda E, Primikiri A, Parpa E. Mystakidou K, Cleeland C, Tsilika E, Katsouda E, Primikiri A, Parpa E, et alGreek MDAnderson Symptom Inventory: validation and utility in cancer patients. Oncology 2004; 67(3-4):203-10. doi: 10.1159/000081318 [Crossref] [ Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5(6):649-55. [ Google Scholar]
- Byrne BM. A Primer of LISREL: Basic Applications and Programming for Confirmatory Factor Analytic Models. Springer Science & Business Media; 2012. 10.1007/978-1-4613-8885-2_1
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling 1999; 6(1):1-55. doi: 10.1080/10705519909540118 [Crossref] [ Google Scholar]
- McDonald RP, Marsh HW. Choosing a multivariate model: noncentrality and goodness of fit. Psychol Bull 1990; 107(2):247-55. doi: 10.1037/0033-2909.107.2.247 [Crossref] [ Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. Vol 210. John Wiley & Sons; 1989. 10.1002/9781118619179
- Higgins PA, Straub AJ. Understanding the error of our ways: mapping the concepts of validity and reliability. Nurs Outlook 2006; 54:23-9. doi: 10.1016/j.outlook.2004.12.004 [Crossref] [ Google Scholar]
- Vilagut G. Test-retest reliability. In: Michalos AC, eds. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer; 2014. p. 6622-5. 10.1007/978-94-007-0753-5_3001
- Bagcivan G, Bredle J, Bakitas M, Guciz Dogan B. Reliability and validity of the Turkish version of the FACIT-PAL quality of life instrument. J Pain Symptom Manage 2019; 58: 297-305.e4. 10.1016/j.jpainsymman.2019.04.020
- Moldón-Ballesteros E, Llamas-Ramos I, Calvo-Arenillas JI, Cusi-Idigoras O, Llamas-Ramos R. Validation of the Spanish versions of FACIT-PAL and FACIT-PAL-14 in palliative patients. Int J Environ Res Public Health 2022; 19(17):10731. doi: 10.3390/ijerph191710731 [Crossref] [ Google Scholar]
- Siegert R, Selman L, Higginson IJ, Ali Z, Powell RA, Namisango E. A psychometric evaluation of the functional assessment of chronic illness therapy-palliative care (FACIT-Pal) scale with palliative care samples in three African countries. J Pain Symptom Manage 2014; 48(5):983-91. doi: 10.1016/j.jpainsymman.2014.01.010 [Crossref] [ Google Scholar]
- Sewtz C, Muscheites W, Kriesen U, Grosse-Thie C, Kragl B, Panse J. Questionnaires measuring quality of life and satisfaction of patients and their relatives in a palliative care setting-German translation of FAMCARE-2 and the palliative care subscale of FACIT-Pal. Ann Palliat Med 2018; 7(4):420-6. doi: 10.21037/apm.2018.03.17 [Crossref] [ Google Scholar]
- Molina B, Rodríguez L, Valdelamar A, Sánchez R. Transcultural adaptation of the scale FACIT-Pal to measure quality of life in patients having advanced cancer in Colombia. Rev Colomb Cancerol 2020; 24(1):18-25. doi: 10.35509/01239015.13 [Crossref] [ Google Scholar]
- Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, Ahles TA. Reliability and validity of the Functional Assessment of Chronic Illness Therapy-Palliative care (FACIT-Pal) scale. J Pain Symptom Manage 2009; 37(1):23-32. doi: 10.1016/j.jpainsymman.2007.12.015 [Crossref] [ Google Scholar]
- Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer 2013; 21(6):1525-50. doi: 10.1007/s00520-012-1688-0 [Crossref] [ Google Scholar]
- Henson LA, Maddocks M, Evans C, Davidson M, Hicks S, Higginson IJ. Palliative care and the management of common distressing symptoms in advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue. J Clin Oncol 2020; 38(9):905-14. doi: 10.1200/jco.19.00470 [Crossref] [ Google Scholar]
- Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res 2012; 21(8):1305-14. doi: 10.1007/s11136-011-0054-x [Crossref] [ Google Scholar]
- Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34(6):557-65. doi: 10.1200/jco.2015.63.0830 [Crossref] [ Google Scholar]
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011; 12(2):160-74. doi: 10.1016/s1470-2045(11)70002-x [Crossref] [ Google Scholar]
- von Blanckenburg P, Leppin N. Psychological interventions in palliative care. Curr Opin Psychiatry 2018; 31(5):389-95. doi: 10.1097/yco.0000000000000441 [Crossref] [ Google Scholar]
- Papadopoulou A, Govina O, Tsatsou I, Mantzorou M, Mantoudi A, Tsiou C. Quality of life, distress, anxiety and depression of ambulatory cancer patients receiving chemotherapy. Med Pharm Rep 2022; 95(4):418-29. doi: 10.15386/mpr-2458 [Crossref] [ Google Scholar]
- Atkin N, Vickerstaff V, Candy B. ‘Worried to death’: the assessment and management of anxiety in patients with advanced life-limiting disease, a national survey of palliative medicine physicians. BMC Palliat Care 2017; 16(1):69. doi: 10.1186/s12904-017-0245-5 [Crossref] [ Google Scholar]
- Merckaert I, Libert Y, Messin S, Milani M, Slachmuylder JL, Razavi D. Cancer patients’ desire for psychological support: prevalence and implications for screening patients’ psychological needs. Psychooncology 2010; 19(2):141-9. doi: 10.1002/pon.1568 [Crossref] [ Google Scholar]
- Cooke PJ, Melchert TP, Connor K. Measuring well-being: a review of instruments. Couns Psychol 2016; 44(5):730-57. doi: 10.1177/0011000016633507 [Crossref] [ Google Scholar]
- Wright EP, Kiely MA, Lynch P, Cull A, Selby PJ. Social problems in oncology. Br J Cancer 2002; 87(10):1099-104. doi: 10.1038/sj.bjc.6600642 [Crossref] [ Google Scholar]
- Ghazzawi A, Kuziemsky C, O’Sullivan T. Using a complex adaptive system lens to understand family caregiving experiences navigating the stroke rehabilitation system. BMC Health Serv Res 2016; 16(1):538. doi: 10.1186/s12913-016-1795-6 [Crossref] [ Google Scholar]